Since the Manchester University of Manchester physicists Andre Geim and Konstantin Novoselov have jointly won the 2010 “Continuity Experiment of Two-dimensional Graphene Materials†After the Bell Physics Prize, any news or research related to graphene has received great attention.
In the past two years, graphene-related industries are also in full swing in China, especially graphene production and production enterprises, such as spring bamboo shoots.
Of course, the international community has not been idle. For example, a sensational news report stated that Graphenano (a company that produces graphene on an industrial scale) cooperates with the University of Corvard in Spain to develop the world’s first graphene polymer battery. The electricity storage capacity is three times that of the best products on the market. Electric vehicles powered by this battery can travel up to 1,000 kilometers and the charging time is less than 8 minutes.
Reading
Graphene was first isolated in 2004. In 2010, the graphene discoverer became known to everyone after winning the Nobel Prize. Today it was only a dozen years. Although the global graphene industry is still in its early stages, due to the public's enthusiasm for new graphene materials, the graphene industry has been overheated and it has shown that it is false to say that the spring breeze of the night is coming and that thousands of trees are blooming. Prosperous scene.
In particular, some areas with relatively abundant graphite mineral resources mix graphene with graphene and regard the development of graphene industry as a “miraculous panacea†for the transformation and upgrading of the local economy. They have planned to construct a graphene industrial park.
Undoubtedly, graphene, as a leader in the new materials industry, is driving the transformation and upgrading of traditional manufacturing industries, nurturing the growth points of emerging industries, and promoting the role of public entrepreneurship and innovation. Under the guidance of the national policy, graphene was laid out in various places. At present, China's entire industry chain of graphene is in its infancy, covering all links from raw materials, preparation, product development, and downstream applications. It has basically formed the Yangtze River Delta, the Pearl River Delta, and the Beijing-Tianjin-Hebei region as a collective area. Development of the graphene industry pattern. In 2016, the overall size of the graphene market in China exceeded 4 billion yuan, and six major market segments have been formed: application in the new energy sector, application in the health field, application in the field of composite materials, application in energy saving and environmental protection, and graphene raw materials and graphene equipment.
However, there is chaos behind the excitement. The momentary prosperity brought only permanent pain. It cannot be mentioned that the current graphene industry in China still faces some deep problems, weak basic research capabilities, lack of leading companies, disconnection of upstream and downstream companies, immature industrial chain, excessive overdraft of graphene in the capital market, and lack of industry standards. And so on, have seriously restricted the healthy and sustainable development of the graphene industry in China.
According to statistics, there are currently more than 40 graphene industrial parks, graphene innovation centers, and graphene research institutes that have been built or under construction in China. More than 2,000 companies are engaged in research and development of graphene raw materials and products, and this figure is still Gradually increase.
It is not advisable for the current vigorous domestic leap-forward “graphene movementâ€. The future graphene industry will be based on the application of graphene materials as a killer, rather than as an all-purpose oil additive. At present, some products on the domestic market, including apparel, coatings, composite materials, adsorption and lubrication products, and graphene lithium battery and graphene mobile phone touch screens, represent the mainstream products of graphene research and development in China, and should be said to be internationally First team. However, compared with foreign countries, we still lag behind. The EU Graphene Flagship Program launched 17 new graphene research projects in October last year. They are concerned about graphene supercars, IoT sensors, wearables, and health. Future frontier areas such as management, data communications, energy technologies, and composites.
Graphene Lithium?
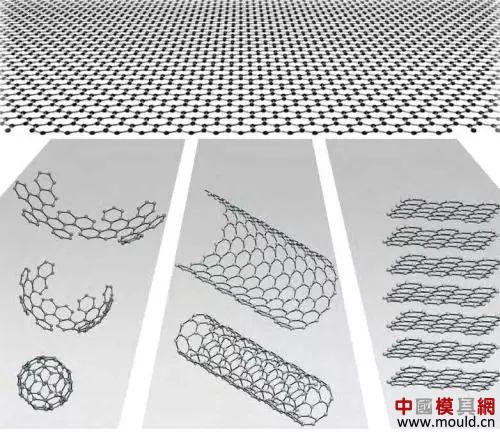
What is graphene? Let's take a look at the definition of Wikipedia: "Graphene" is a planar film consisting of carbon atoms in the form of sp2 hybrid orbitals consisting of a hexagonal honeycomb lattice, a two-dimensional material with only one carbon atom thickness. Graphene is currently It is the thinnest but hardest nanomaterial in the world. It is almost completely transparent and only absorbs 2.3% of light. Its thermal conductivity is as high as 5300 W/m·K, higher than carbon nanotubes and diamonds, and its electron mobility at room temperature. More than 15000 cm2/V·s, higher than carbon nanotubes or silicon crystals, and the resistivity is only about 10-8 μm, which is lower than that of copper or silver and is the smallest material in the world.
The current term "graphene battery" is very hot. In fact, there is no reference to "graphene battery" in the international lithium academia and industry. The author searched Wikipedia and found no explanation for the terms "graphene battery" or "graphene Li-ion battery."
According to Graphene-info, the authoritative Graphene website of the United States, the definition of "graphene battery" is the addition of a graphene material to the electrode material. This explanation is obviously misleading. According to the classic electrochemical nomenclature, lithium-ion batteries used in smartphones in general should be named "lithium cobaltate-graphite batteries."
The reason why it is called "lithium-ion battery" is because when SONY put lithium-ion battery on the market in 1991, considering that the classical nomenclature is too complicated, people can't remember it, and the charge-discharge process is realized through the migration of lithium ions. The system does not contain metallic lithium, so it is called "Lithium ion battery." In the end, the name “lithium-ion battery†was widely accepted around the world, which also reflects the special contribution of SONY in the field of lithium power.
At present, almost all commercial lithium-ion batteries use graphite anode materials. In the case of similar anode performance, the performance of lithium-ion batteries largely depends on the cathode material, so now lithium-ion batteries also have the habit of calling them in accordance with the cathode. . For example, lithium iron phosphate batteries (BYD's so-called "iron batteries" are not in the scope of this article), lithium cobalt oxide batteries, lithium manganese batteries, triple batteries, etc., are for the cathode.
Then if the battery negative electrode with silicon material, will not be called silicon battery? Maybe it is possible. But no matter what, whoever plays a major role is named after whom. According to this calculation, if you want to call the graphene battery must be the main electrochemical effect of graphene cells. It's like a lithium cobalt oxide battery with carbon black. Can't call it a carbon black battery? In order to further clarify the concept of "graphene battery", we first summarize the possible applications of graphene in lithium-ion batteries (only possible).
Negative electrode: 1. Graphene alone is used for a negative electrode material; 2. A composite material is formed with other new negative electrode materials such as silicon-based and tin-based materials and transition metal compounds; 3. Anode negative electrode conductive additive.
• Positive electrode: Mainly used as a conductive agent added to lithium iron phosphate positive electrode to improve the rate and low temperature performance; also added to the study of lithium manganese phosphate and lithium vanadium phosphate to improve the cycle performance.
· Graphene functional coating aluminum foil, its actual performance with ordinary carbon coated aluminum foil (A123 combined with Henkel Development) has not much improved, on the contrary, the cost and complexity of the process increased a lot, the possibility of commercialization of the technology is very low.
From the above analysis, it is clear that there are only two areas in which graphene may play a role in lithium-ion batteries: direct use in anode materials and in conductive additives.
The industrialization of lithium anodes is still difficult.
We first discuss the possibility of using graphene alone as a lithium anode material. The charge-discharge curves of pure graphene are very similar to those of hard carbon and activated carbon materials with high specific surface areas. They all have the disadvantages of extremely low first-cycle coulombic efficiency, excessively high charge and discharge plateaus, severe potential lag, and poor cycle stability. These problems are in fact all problems. It is a basic electrochemical feature of a high specific surface disordered carbon material.
High-quality graphene has very low tapping and compaction densities and is extremely expensive, and there is no possibility of directly replacing the graphite material as a negative electrode of a lithium-ion battery. Since it is not feasible to use graphene alone as a negative electrode, what is the graphene composite negative electrode material?
Graphene and other new anode materials, such as silicon-based and tin-based materials and transition metal compounds form a composite material, is currently the most popular research area of ​​"Lithium-Ion-Lithium", and thousands of papers have been published in the past few years. The principle of compounding is to use the flexibility of graphene sheets to buffer the volume expansion of these high-capacity electrode materials during the cycle. On the other hand, the excellent conductivity of graphene can improve the electrical contact between the particles and reduce the polarization. All of these factors can improve the electrochemical performance of composites.
However, it is not said that only graphene can achieve the improvement effect, and practical experience shows that similarly or even better electrochemical performance can be obtained by using a conventional carbon material composite technology and process. For example, Si/C composite negative electrode material, compared with ordinary dry composite technology, composite graphene does not significantly improve the electrochemical performance of the material, but instead increases the complexity of the process due to the dispersion and compatibility problems of graphene. It affects batch stability.
If comprehensive consideration is given to material cost, production process, processability, and electrochemical performance, graphene or graphene composites are unlikely to be practically used for lithium negative electrodes. Industrialization prospects are difficult.
There is no obvious advantage as a conductive agent.
Let us say that graphene is used as a conductive agent. Nowadays, conductive materials used in lithium batteries are conductive carbon black, acetylene black, Ketchen black, and Super P. Nowadays, there are also battery manufacturers that have begun to use carbon fiber (VGCF) on power batteries. And carbon nanotubes (CNT) as a conductive agent.
The principle of graphene as a conductive agent is its excellent electron transport capability due to its special structure of two-dimensional high specific surface area. From the current accumulated test data, VGCF, CNT, and graphene all have a certain increase in rate performance compared to Super P. However, the difference in the degree of electrochemical performance improvement between the three is small, and graphene does not Shows obvious advantages.
Is it possible that the addition of graphene can cause the electrode material to explode? The answer is very embarrassing. Taking the iPhone cell phone battery as an example, the increase in battery capacity is mainly due to the increase in the LCO operating voltage, which raises the upper limit charging voltage from 4.2V to 4.35V on the current i-Phone 6, making the LCO capacity from 145 mAh/g. Gradually increase to 160-170mAh/g (high-pressure LCO must be modified by bulk doping and surface coating), and these improvements have nothing to do with graphene.
In other words, if you use a high-voltage lithium cobalt oxide with a cut-off voltage of 4.35V and a capacity of 170mAh/g, how much you add graphene cannot increase the capacity of lithium cobalt oxide to 180mAh/g, not to mention that it is several times faster. The so-called "graphene battery" capacity. Can the addition of graphene improve the cycle life of the battery? This is also embarrassing. Graphene has a larger specific surface area than CNTs. Adding more negative ions to the negative electrode can only generate more SEI and consume lithium ions. Therefore, CNTs and graphene can only be added to the positive electrode to improve the magnification and low temperature performance.
What about the cost? At present, the production cost of high-quality graphene is still expensive, and the so-called inexpensive “graphene†products on the market are basically graphite nanosheets (the proportion of the layer with more than ten layers is large).
If we compare graphene and CNT, we will find that the two have striking similarities, all have a lot of almost exactly the same "exotic performance", when the "magic performance" of CNT is now fully applied to the graphite On the body. The CNTs began to heat up internationally at the end of the last century and reached a climax between 2000 and 2005. CNTs are said to have very many functions and there are also many "unique characteristics" in the field of lithium batteries.
However, more than 20 years have passed, and it has not been seen so far that these “exotic properties†of CNTs are actually applied in large-scale applications. In terms of lithium, CNT is only used as a positive electrode conductive agent. A small-scale trial was started in the LFP power battery in the past two years (the cost performance is still lower than that of VGCF), and LFP power battery has been destined to become the mainstream technology route for electric vehicles.
Compared with CNTs, graphene is very similar in electrochemical performance and does not have any special features. On the contrary, the production cost is higher, the production process is more serious to the environment, and the actual operation and processing performance are more difficult. The current so-called "graphene batteries" are purely hype, and there are only a few that really calm down, most of them taking the "fast food economy" route. Comparing CNT and graphene, "history is always the same!"
Where is the real application of graphene?
Future prospects for the use of graphene in lithium-ion batteries are difficult. Compared with lithium-ion batteries, the application prospects of graphene in supercapacitors, especially micro-supercapacitors, seem to be a little bit trickier, but we still have to be wary of some academic hype.
In fact, looking at a lot of these so-called "academic breakthroughs," you will find that many professors in the paper intentionally or unintentionally confused some basic concepts. The current commercial activated carbon supercapacitors typically have an energy density of 7-8 Wh/kg, which refers to the device energy density of the entire supercapacitor containing all components. The breakthroughs mentioned by the professors generally refer to the energy density of the material, so actual graphene supercharging is not as good as mentioned in the paper.
In contrast, the cost requirements of micro-supercapacitors are not as stringent as those of ordinary capacitors. Graphene composites are used as electrochemically active materials, and the appropriate ionic liquid electrolytes are selected. It is possible to achieve the duality of both conventional capacitors and lithium-ion batteries. Advantageous energy storage devices may be (only possible) in certain niche areas such as micro-electromechanical systems (MEMS).
Metal assembly refers to the process of joining two or more metal
components to create a single, functional unit. This process can be
accomplished through a variety of methods, including welding, brazing,
soldering, and mechanical fastening.
Welding involves melting the metal components at the joint and fusing
them together. This can be done using various heat sources, including
gas flames, electric arcs, and lasers. Welding is commonly used in the
construction of buildings, bridges, and other large structures.
Brazing and soldering are similar processes that involve melting a
filler material to join the metal components. Brazing uses a
high-temperature filler material, while soldering uses a
lower-temperature material. These processes are often used in the
manufacture of electronics and plumbing components.
Mechanical fastening involves using bolts, screws, or other fasteners to
hold the metal components together. This method is commonly used in the
assembly of machinery and vehicles.
Metal assembly is a critical process in many industries, including
manufacturing, construction, and transportation. It requires skilled
technicians and specialized equipment to ensure that the components are
joined correctly and that the final product meets the required
specifications.
Metal Assembly,Zinc Electroplating Metal Plate,Nickel Plated Metal Plate,Perforated Plate Stamping
Suzhou Gold ant Precision Sheet Metal Co.,Ltd , https://www.jmysheetmetal.com
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)